
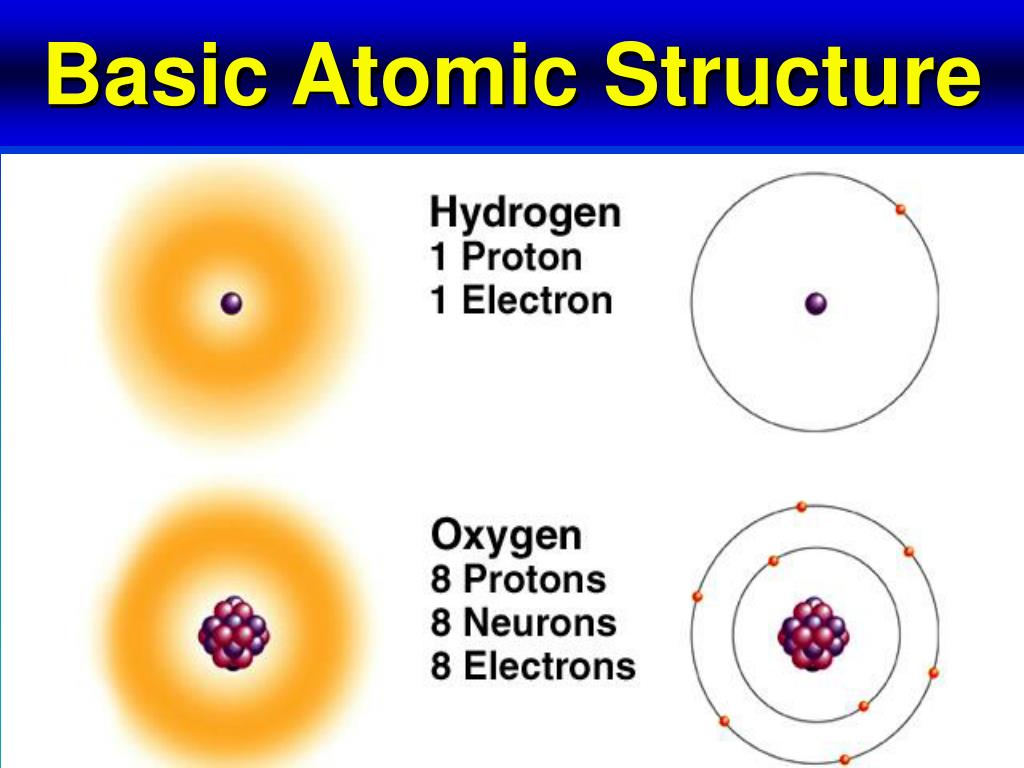
The amu was originally defined based on hydrogen, the lightest element, but now is defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). For example, a carbon atom weighs less than 2 × 10 −23 g, and an electron has a charge of less than 2 × 10 −19 C (coulomb). (credit middle: modification of work by “babyknight”/Wikimedia Commons credit right: modification of work by Paxson Woelber)Ītoms-and the protons, neutrons, and electrons that compose them-are extremely small. If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium ( Figure 1)! Figure 1. The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m-about 100,000 times smaller.
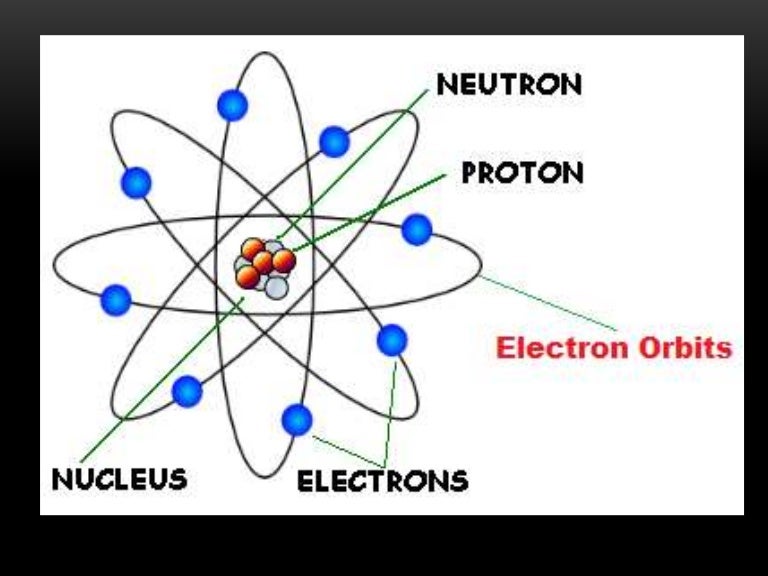
The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The development of modern atomic theory revealed much about the inner structure of atoms. Define the atomic mass unit and average atomic mass.Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
